The Development, Implementation and Assessment of Novel Training in Domain-based Competencies, or “DIAMOND” project, is a collaborative effort by the University of Michigan, University of Rochester, The Ohio State University and Tufts University to provide competency-based education and training to clinical research professionals. The primary objectives of this project are to develop an online educational portal for shared competency-based educational offerings and assessments, demonstrate the utility of an e-portfolio system for individualized professional development planning, and to disseminate these educational offerings to broader audiences at research institutions across the United States.
Project Overview
Successful clinical and translational research projects require a well-prepared, competent workforce of clinical research professionals. Clinical research professionals (CRPs) include clinical research coordinators and others who are team members facilitating clinical research projects, including junior investigators. The need for a competent clinical research professional workforce sparked a movement by the Enhancing Clinical Research Professionals’ Training & Qualification (ECRPTQ) project team to select and modify the Joint Task Force Framework (eight competency domains) to better define the knowledge, skills and abilities (KSAs) for this profession. The DIAMOND project has created a federated educational portal to house links to shared educational materials and assessments, sorted by domain. DIAMOND will fill an educational gap by providing the CTSA consortium with an accessible training and workforce development strategy for the CRP workforce for higher quality and safer clinical trial execution.
DIAMOND Specific Aims
- Specific Aim 1: Create an online portal containing CTSA-shared resources and strategies used to train clinical research professionals which are mapped to clinical trial core competencies Study Activities:
- Develop a discovery learning space (DIAMOND portal)
- Develop the DIAMOND curriculum by continuing to identify and map educational offerings to the competency domains. Create educational offerings for domains for which suitable training is currently not available.
- Demonstrate use of an ePortfolio system which allows users to track their DIAMOND training
- Specific Aim 2: Develop a validated set of assessment tools designed to evaluate the ECRPTQ core competencies for the CRP workforce Study Activities:
- Identify and validate currently available competency assessments for use in clinical trials training
- Develop new competency-based assessments that demonstrate an applied learning and skill development
- DIAMOND Project Timeline:
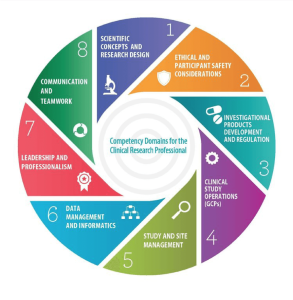
- Scientific concepts and research design: Knowledge of scientific concepts related to the design and analysis of clinical trials
- Ethical and participant safety considerations: Care of patients, aspects of human subject protection and safety in the conduct of a clinical trial
- Medicines development and regulation: Knowledge of how drugs, devices, and biologicals are developed and regulated
- Clinical trial operations: Study management, GCP compliance, safety management, and handling of investigational product
- Study and site management: Site and study operations
- Data management and informatics: How data are acquired and managed during a clinical trial
- Leadership and professionalism: The principles and practices of leadership and professionalism in clinical research
- Communication and teamwork: All elements of communication within the site and between the site and sponsor. Teamwork skills necessary for conducting a clinical trial.
Helpful tools and instructional guides to help you make the most of the DIAMOND Portal and e-Portfolio.
- Self-Assessment Tool for Professional Development – Clinical research professional core competencies self-assessment for each of the 8 ECRPTQ domains.
- ECRPTQ Competencies – A brief description of the competency domains that are included within the Enhancing Clinical Research Professionals’ Training and Qualifications (ECRPTQ) framework.
